|
The
basis for palaeoclimatic interpretations of variations in the stable isotope
content of water molecules is that the vapour pressure of H216O
is higher than that of H218O. Evaporation from a
water body thus results in a vapour which is poorer in 18O
than the initial water; conversely, the remaining water is enriched in
18O. During condensation, the lower vapour pressure of the
H218O ensures that it passes more readily into the
liquid state than water vapour made up of the lighter oxygen isotope.
During the poleward transportation of water vapour, such isotope fractionation
continues this preferential removal of the heavier isotope, leaving the
water vapour increasingly depleted in H218O. Because
condensation is the result of cooling, the greater the fall in temperature,
the lower the heavy isotope concentration will be. Isotope concentration
in the condensate can thus be mainly considered as a function of the temperature
at which condensation occurs. Water from polar snow will thus be found
to be most depleted in H218O. Overall, however,
also other effects determine the isotopic composition.
In Figure 1, oxygen isotopic records from different regions
are shown. Please note that the values are less depleted in 18O
during the last 10'000 years (Holocene) than before (Late Glacial Time
and Last Glacial Maximum). This indicates that the Holocene is a warmer
period.
|
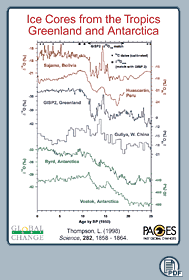
1 - Ice cores from the tropics, Greenland and Antarctica,
showing isotope data. (184K)
|

 There
are three stable isotopes of Oxygen, 16O, 17O, 18O,
in the proportion of 99.76%, 0.04% and 0.2% respectively. 16, 17 and 18
refer to the number of nucleons contained within the O Atom. An atom of
each isotope contains 8 protons. It is the number of neutrons, which varies.
Consequently, 18O is heavier than 17O, which is heavier
than 16O.
There
are three stable isotopes of Oxygen, 16O, 17O, 18O,
in the proportion of 99.76%, 0.04% and 0.2% respectively. 16, 17 and 18
refer to the number of nucleons contained within the O Atom. An atom of
each isotope contains 8 protons. It is the number of neutrons, which varies.
Consequently, 18O is heavier than 17O, which is heavier
than 16O. 