|
|
|
|
|
|
|
During the cold winter months precipitation in higher elevations and higher latitudes usually falls in the form of snow. The water-saturated air cools down to below 0°C, and consequently the excessive humidity sublimates to ice crystals. Dust particles such as clay minerals in the air act as nucleating agents to freeze cloud droplets. We distinguish two processes of nucleation (crystallisation): Heterogeneous and homogeneous nucleation. These two processes are shown in the animation below. |
1 - Alpine landscape in snow (140K) |
|||||
|
Let it snow! Look at the differences between the processes of heterogeneous and homogeneous nucleation. Try to explain the processes to another student. |
||
|
During precipitation, ice crystals associate to form snow flakes with different forms and sizes. The ice crystals forming snow flakes are only distinguishable under a microscope. Their shapes depend on temperature. |
|
Accumulating on the ground, the seasonal snow cover has a variety of physical and chemical characteristics important for the biotic environment: Snow |
|
|
2 - A young spruce tree bending under the load of a settling and creeping snow cover (32K) |
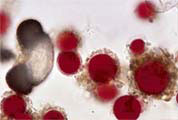
3 - Red resting spores of the snow alga Chlamydomonas nivalis in a snow probe (to the left a pollen grain of Pinus) (80K) |
4 - This faint red areas are derived from snow algae. (76K) |
5 - An Ibex fighting its way through the snow (80K) |
| Snow movements such as snow
gliding and avalanches redistribute snow and break higher woody
plants thus influencing the structure of vegetation (Fig. 6). |
6 - Bent and broken trees in the runout zone of an avalanche
(164K) |
| The difference between the influence
of snow cover on the environment in polar regions and its influence in
high mountains of temperate and tropical regions is mainly due to differences
in temperature. Here, we focus on the snow cover of temperate mountains,
especially the Alps.
|
|
29 August 2011 |
||
| |
||